Lupine Publishers | Ovarian Mucinous Borderline Tumor with Malignant Muralnodules: A Report of Two Cases
Lupine Publishers | Interventions in Gynecology and Women's Healthcare
Keywords: Mucinous ovarian tumor; Mural nodule; Anaplastic carcinoma; Angiosarcoma; Poorly differentiated carcinoma; Poor prognosis
Introduction
Mucinous tumors of the ovary account for about 15% of all ovarian tumors; 80% are benign, while only 3-4% are invasive carcinoma; the remainder are borderline tumors [1]. Rarely, mucinous tumors may exhibit mural nodules containing a carcinomatous or sarcomatous component; these mural nodules are more commonly associated with mucinous borderline tumors but can also arise within mucinous carcinoma [1, 2]. Prat and Scully first described mucinous ovarian tumors with mural nodules in a series of 7 cases in 1979; these lesions were termed mucinous tumors with sarcoma-like nodules [3,4]. However, classification of mural nodules arising in mucinous ovarian tumors remains unclear, and several authors have proposed different classification systems [5-14]. Anaplastic carcinoma is the most common type of mural nodular malignancy; these tumors can infiltrate borders and invade blood vessels; they readily metastasize and generally have a poor prognosis [6,7]. We describe two cases of postmenopausal women with malignant mural nodules arising from mucinous borderline tumors of the ovary.
Case reports
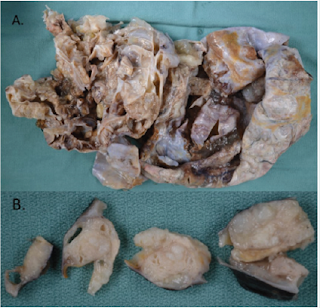
Figure 1: A Photograph showing ovarian mass containing mural nodules, B: Mural nodules observed in wall of ovarian mass.
Case 1: A 58-year-old female presented with a several month history of weight gain and abdominal discomfort; CT scan of the abdomen and pelvis demonstrated a large pelvic mass arising from the uterus and right adnexa, with septations and enhancing components. Preoperative tumor markers were elevated: CA-125 at 367, and CA19-9 at 2266. Peritoneal ascitic fluid contained atypical cells suspicious for malignancy. Hysterectomy, bilateral salpingooophorectomy, and omentectomy were performed. Grossly, the ovarian lesion was 31.0cm x 29.0 cm x 15.0cm in size. Capsule was collapsed, and mucoid material was observed oozing from the surface, which was otherwise whitish and smooth; cut surfaces revealed multiloculation, with clear mucinous material (Figure 1A). Several solid areas consisting of firm, tan-white, somewhat mucoid tissue were noted in the wall of the ovarian mass (Figure 1B). Histology revealed a complex mucinous neoplasm with benign, borderline, and malignant change, containing multiple solid nodules of anaplastic carcinoma (Figure 2).
Figure 2: Microscopic appearance of ovarian tumor (hematoxylin & eosin staining). A. Mucinous borderline tumor, B. Mucinous borderline tumor with mural nodule containing focus of anaplastic carcinoma, admixed with discrete borders.
Figure 3: Microscopic appearance of mural nodule containing anaplastic carcinoma (hematoxylin & eosin staining). Tumor cells showed eosinophilic cytoplasm, high grade eccentric nuclei, and prominent nucleoli.
A small focus of intraepithelial carcinoma was identified within the mucinous borderline tumor. Tumor cells had eosinophilic cytoplasm, high grade eccentric nuclei, and prominent nucleoli; some areas showed spindle cell proliferation and rhabdoid differentiation (Figure 3). The ovarian surface was involved by anaplastic carcinoma; this component was also present in lymphovascular spaces in the left fallopian tube, paratubal soft tissue, and paradnexal tissue. A macroscopic extraperitoneal focus of anaplastic carcinoma was detected in the omentum. The anaplastic carcinoma component showed diffuse immunopositivity for CK7, and weak positivity for CK20; p53 was diffusely positive (Figure 4). The mucinous borderline component was also positive for CK7 and CK20, as well as PAX8, CDX2, and villin. In both components, p16 showed mosaic staining, and ER and PR were negative.
Figure 4: Immunohistochemical features of ovarian lesion, showing mucinous borderline tumor and mural nodule with anaplastic carcinoma. Both the mucinous borderline and anaplastic carcinoma components showed diffuse immunopositivity for CK7 (A), the anaplastic component showed weak positivity for CK20, while the borderline component was strongly positive for CK20 (B); both components showed mosaic staining for p16 (C), anaplastic component was diffusely positive for p53 (D).
Case 2: A 52-year-old female presented with a two-month history of abdominal discomfort, bloating, and weight gain. CT scan of the abdomen and pelvis showed a large cystic mass with internal septations and several solid components, favoured to arise from the right ovary. Preoperative CA-125 was slightly elevated at 42. Peritoneal ascitic fluid was negative for malignant cells. Hysterectomy, bilateral salpingo-oophorectomy, omentectomy, appendectomy, and peritoneal biopsies were performed. Grossly, the ovarian lesion was 30.0 cm x 18.0 cm x 15.0 cm in size with a smooth, intact capsule, and one 40 mm solid nodule, which consisted of tan, hemorrhagic, friable, multicystic tissue (Figure 5). Microscopically, a mucinous borderline tumor with intraepithelial carcinoma and a mural nodule was observed. The nodule was predominantly composed of pleomorphic spindle cells, but also had an epithelial component (Figure 6). Spindle cells contained hyperchromatic nuclei and were arranged in a vaguely fascicular pattern with some vascular-like spaces; no vascular invasion was seen (Figure 6).
Figure 5: Photograph showing ovarian lesion containing a solid nodule.
Figure 6: Microscopic appearance of ovarian tumor (hematoxylin & eosin staining). A. Mucinous borderline tumor. B. Mucinous borderline tumor with mural nodule containing pleomorphic spindle cells. C. Mural nodule containing pleomorphic spindle cells and poorly differentiated carcinoma. D. Angiosarcoma component.
Figure 7: Immunohistochemical features of ovarian lesion, showing a small focus of mucinous borderline tumor and mural nodule with poorly differentiated carcinoma. Both the mucinous borderline and poorly differentiated carcinoma components showed diffuse immunopositivity for CKAE1/AE3 (A), and CAM5.2 (B).
Figure 8: Immunohistochemical features of ovarian lesion, showing mural nodule with spindle cell component consistent with angiosarcoma, which showed diffuse immunopositivity for vimentin (A), CD31 (B and C), and ERG (D).
Immunohistochemical stains for CK AE1/AE3 and CAM5.2 highlighted the mural nodule’s epithelial component, which was consistent with invasive poorly differentiated carcinoma (Figure 7). The spindle cell component was negative for pancytokeratins, and diffusely positive for vimentin, CD31 and ERG; consistent with angiosarcoma (Figure 8). The case was sent for outside consultation, and the mural nodule was determined to be a malignant spindle cell neoplasm consistent with angiosarcoma with admixed foci of poorly differentiated carcinoma. Ultimately, the mural nodule was classified as a malignant mesodermal mixed tumor (carcinosarcoma).
Discussion
Mucinous borderline tumors of the
ovary containing malignant mural nodules are rare entities; a small number of
cases have been described since 1979 [6,8,9,14]. Prat and Scully (1979)
proposed a classification system dividing mural nodules into three classes based
on histology: type 1 nodules (pleomorphic and epulis like), type 2 nodules
(pleomorphic and spindle cell) and type 3 nodules (giant cell-histiocytic) [3].
Other reports have described mural nodules based on gross examination:
sarcoma-like, which are typically multifocal and contain large areas of
hemorrhage, anaplastic or undifferentiated carcinomas, which are often
multifocal but less hemorrhagic than sarcoma-like mural nodules; and sarcoma,
which tend to be solitary nodules [10-12]. Additional reports have classified
mural nodules into five or six groups: anaplastic carcinoma, sarcoma,
sarcoma-like, mixed, and smooth muscle sarcoma; carcinosarcoma may be
considered a grouping, as well as leiomyoma [1,5,14,15].
In our two cases, the patients were close in age and presented with similar
symptoms: weight gain and abdominal discomfort. Imaging of both ovarian lesions
showed similar large cystic pelvic masses with septations. Gross examination of
the lesions also revealed similarities: both lesions were over 30cm in greatest
dimension, multiloculated, and contained solid nodules within the wall.
Microscopic features of the mural nodule in case 1 were easy to discern, given
the eosinophilic cytoplasm, high grade eccentric nuclei, with focal spindle
cell proliferation, which are characteristic of anaplastic carcinoma. The mural
nodule in case 2, however, had both a spindle cell and epithelial component and
required expert consultation to diagnose as angiosarcoma with poorly
differentiated carcinoma. Therefore, the mural nodule in case 2 may be
categorized as carcinosarcoma, mixed, or sarcoma, depending on which
classification scheme is referred to. Prognosis for patients with mural nodules
arising from mucinous borderline tumors of the ovary is generally thought to be
poor, especially for sarcomatous and anaplastic mural nodules [7-13].
A more favorable prognosis is expected in patients where the malignant mural
nodule is confined to the ovary and/or lacks vascular invasion [9-15]. In
addition, type 3 nodules (giant cellhistiocytic) in the classification proposed
by Prat and Scully (1979) are also thought to behave in a benign manner and
therefore portend a good prognosis [3]. However, a larger study of 34 cases of
anaplastic carcinoma arising in mucinous ovarian tumors concluded that foci of
anaplastic carcinoma in unruptured stage I mucinous tumors may not foretell an
unfavorable prognosis; one third of patients who were followed up died within 8
months, but these were stage >1C tumors; only one patient with stage 1A
disease died [14]. In case 2, mural nodules had not disseminated beyond the
ovary, and no lymphovascular invasion was identified. The patient remains free
of local recurrence or metastases approximately 3 years after surgery; she
continues to undergo active surveillance every 6 months with CT scans. The
patient in case 1 is only a few months post operative and is currently disease
free despite the presence of lymphovascular invasion and dissemination of the
anaplastic component to the ipsilateral fallopian tube, paratubal soft tissue,
paradnexal tissue, and omentum (pT3c). She is currently receiving chemotherapy
with Carboplatin and Paclitaxol. A Lynch Study demonstrated intact staining for
both the mucinous borderline tumor and anaplastic carcinoma component in this
lesion.
Conclusion
We present two cases of a rare entity: mural nodules arising in a mucinous borderline tumor of the ovary. In case 1, anaplastic carcinoma arose in a nodule, and metastases of this primary neoplasm were observed diffusely around the pelvis; precluding the fact that anaplastic carcinoma within a mucinous neoplasm leads to aggressive behaviour. Case 2 was unusual since two different malignant components were observed within the mural nodule. There is conflicting evidence in the literature regarding progression of mural nodules; our first case was limited to the ovary, and the patient experienced a favourable outcome; the second case was an anaplastic mural nodule that disseminated around the pelvis, and the patient is still undergoing treatment; these results seem to fit with past reports detailing behaviour of these tumors. Ultimately, close follow up of patients with mural nodules arising in mucinous borderline tumors of the ovary is imperative given the tendency of these lesions to spread to lymphovascular spaces and metastasize. In addition, progression of malignant neoplasms arising in mucinous borderline tumors should be included in the differential diagnosis when undifferentiated tumors are identified in the omentum and the patient has a history of a pelvic mass.
Read More Lupine Publishers Gynecology Journal Articles: https://lupinepublishers-gynecology.blogspot.com/


Comments
Post a Comment